Current projects
Transfer learning approaches for reaction product prediction in the low-data regime
Graduate projects
Undergraduate projects
Papers
Peer-reviewed academic papers. Go to my Google Scholar page for citation data.
Working manuscripts & preprints
- Nathan M. Lui*, Pushkar G. Ghanekar, Matthew A. Schiffler & W. Dean Harman “Transfer learning approaches for reaction product prediction in the low-data regime.” Manuscript in preparation *Corresponding author
- Nathan M. Lui*, Max Li & Matthew Ford “MoFlowGAN: Combining adversarial and likelihood learning to enable targeted molecular generation.” ChemRxiv Preprint 2023. Preprint. Code. *Corresponding author
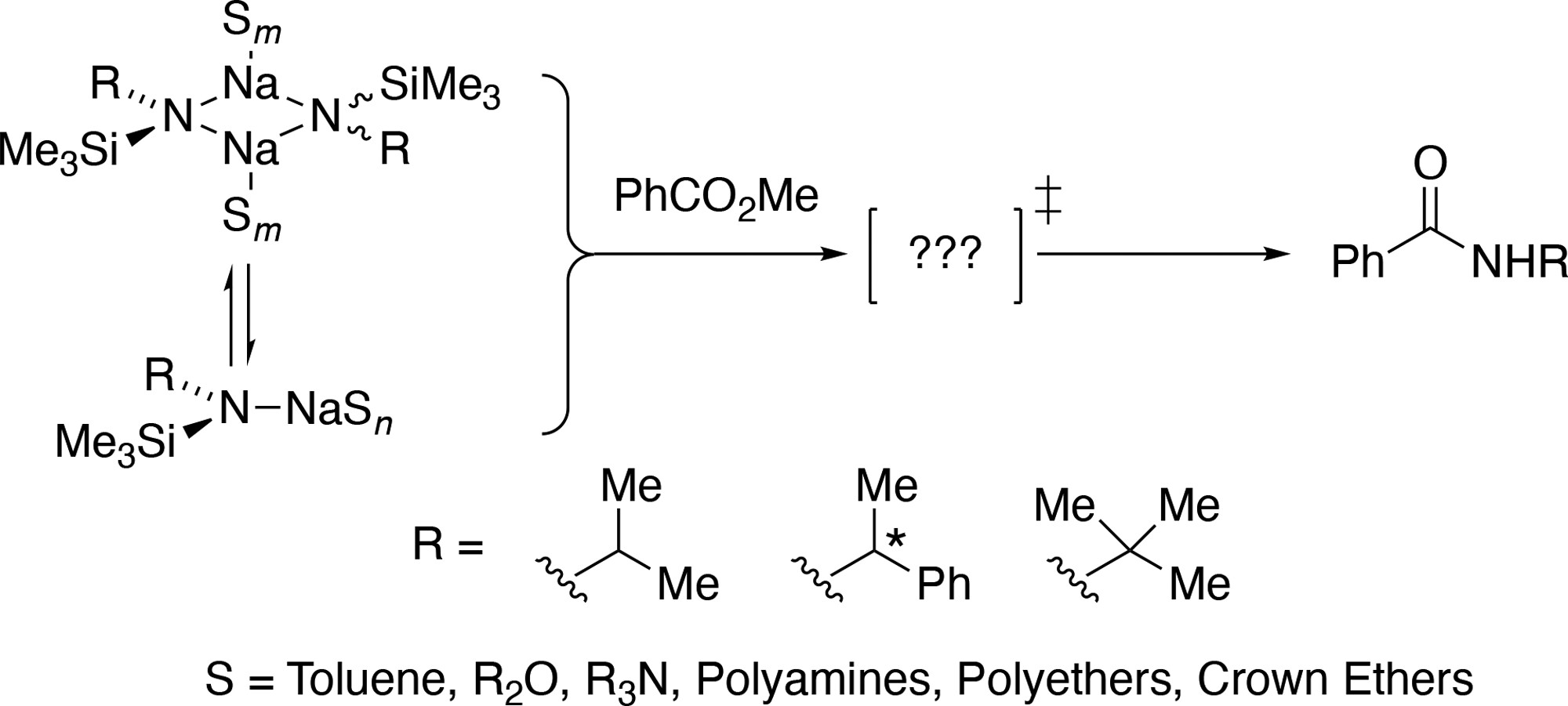
Sodium Alkyl(trimethylsilyl)amides: Substituent- and Solvent-dependent Solution Structures and Reactivities
Qiulin You, Yun Ma, Ryan A. Woltornist, Nathan M. Lui , Jesse A. Spivey, Ivan Keresztes & David B. Collum
Abstract
The preparation of sodium isopropyl(trimethylsilyl)amide (NaPTA), sodium (1-phenylethyl)(trimethylsilyl)amide (NaPETA), sodium tert-butyl(trimethylsilyl)amide (NaBTA), and isotopologues [15N]NaPTA and [15N]NaBTA are described. Solution structural studies using a combination of 29Si NMR spectroscopy, the Method of Continuous Variations, and density functional theory computations provided insights into aggregation and solvation in a range of solvents including toluene, N,N-dimethylethylamine, triethylamine, MTBE, THF, 1,2-dimethoxyethane (DME), diglyme, N,N,N',N'-tetramethylethylenediamine (TMEDA), N,N,N',N'-tetramethylcyclohexanediamine (TMCDA), N,N,N',N',N'-pentamethyldiethylenetriamine (PMDTA). 12-crown-4, 15-crown-5, and 18-crown-6 revealed solvent- and substituent-dependent dimer–monomer mixtures with affiliated solvation numbers. Complexation of the three crown ethers documented both crown and substituent dependencies. Qualitative studies of reactivity showed a variety of reactions of NaPETA. Aminolysis of methyl benzoate with dialkylamines mediated by NaPTA afforded high yields of benzamides. Quantitative rate studies of aminolysis of methyl benzoate by NaPTA revealed a 47,000-fold range of rates. Detailed rate studies in toluene and THF showed dimer-based mechanisms. The role of primary- and secondary-shell solvation by THF is discussed, including nuances of methods used to separate the two contributions. PMDTA-solvated NaPTA monomer reacts as a monomer whereas bis-diglyme solvated monomer reacts as a dimer. Rate studies exploring the structure–reactivity correlations of the three crown ethers show mono- and bis-crown-based pathways in which 15-crown-5─the crown ether often said to be of choice for sodium─was decidedly inferior as an accelerant.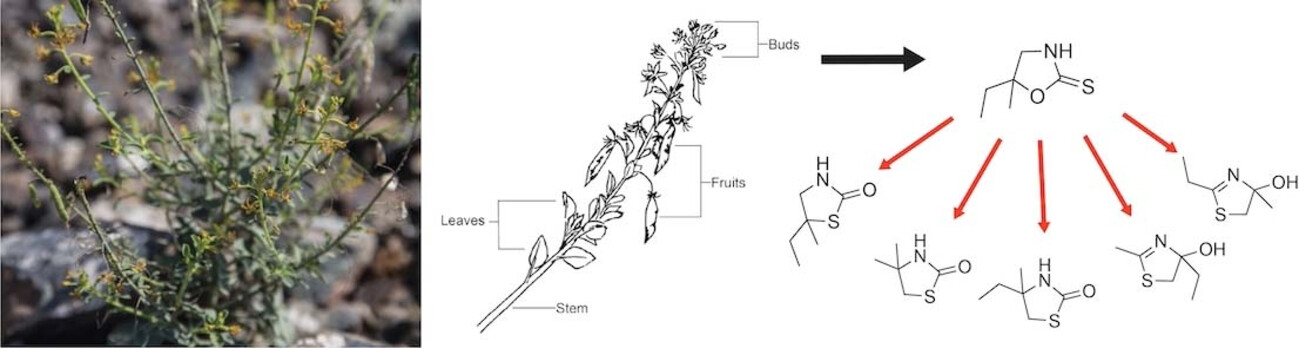
Natural Product Isolation of the Extract of Cleome rupicola Fruits Exhibiting Antioxidant Activity
Yumi Gambrill, Patrick Commins, Stefan Schramm, Nathan M. Lui, Shaikha S. AlNeyadi & Panče Naumov
Abstract
Cataracts are the leading cause of blindness worldwide, however, there is currently no drug-based treatment. Plants that exhibit antioxidant properties have shown promising anticataract effects, likely because they supplement the activity of glutathione, the major antioxidant in lens cells. An extract of Cleome rupicola, a desert plant found in the United Arab Emirates, has traditionally been used to treat cataracts. Phytochemical screening of the aqueous extract established the presence of flavonoids, tannins, steroid derivatives, and reducing sugars. Fractioning of extracts from the fruits using high-performance liquid chromatography (HPLC) yielded the isolation of the anthelmintic compound cleomin, and its structure was confirmed using mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy.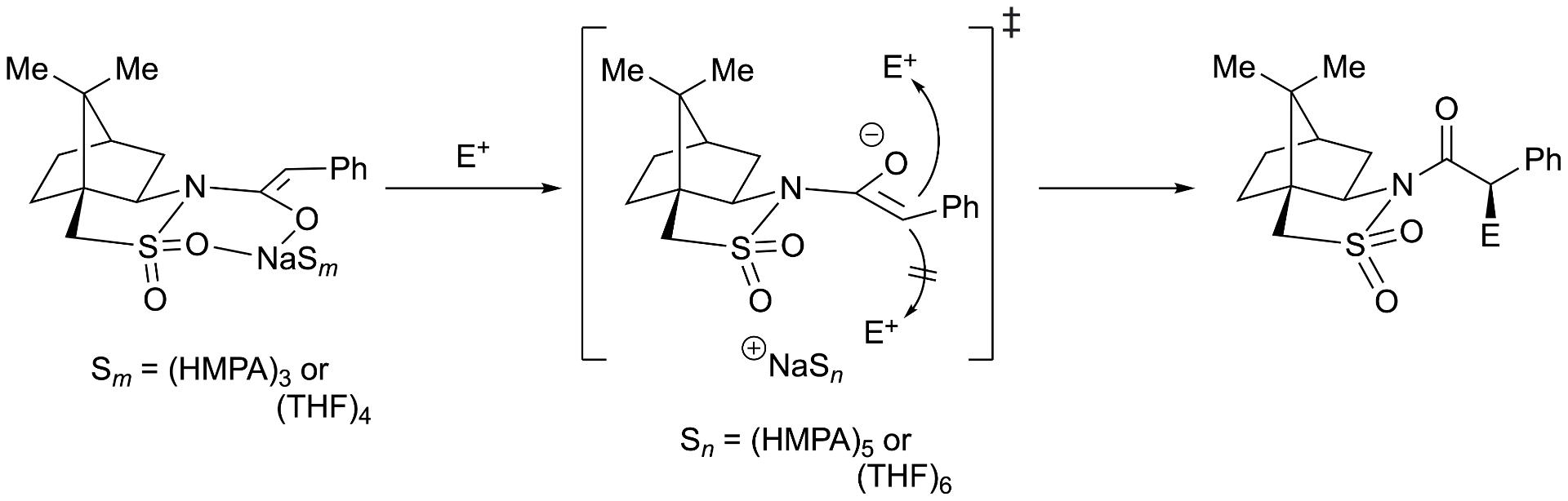
Sodiated Oppolzer enolates: solution structures, mechanism of alkylation, and origin of stereoselectivity
Nathan M. Lui & David B. Collum
Accolades
Featured in the 2023 HOT articles collection of Organic Chemistry FronitersAbstract
NMR spectroscopic studies reveal camphorsultam-derived sodium enolates known as Oppolzer enolates reside as monomers in neat THF and THF/HMPA solutions and as dimers in toluene when solvated by N,N,N′,N′-tetramethylethylenediamine (TMEDA) and N,N,N′,N′′,N′′-pentamethyldiethylenediamine (PMDTA). Density functional theory (DFT) computations attest to the solvation numbers. Rate studies show analogy with previously studied lithiated Oppolzer enolates in which alkylation occurs through non-chelated solvent-separated ion pairs. The origins of the selectivity trace to transition structures in which the alkylating agent is guided to the exo face of the camphor owing to stereoelectronic preferences imparted by the sultam sulfonyl moiety. Marked secondary-shell solvation effects are gleaned from the rate studies.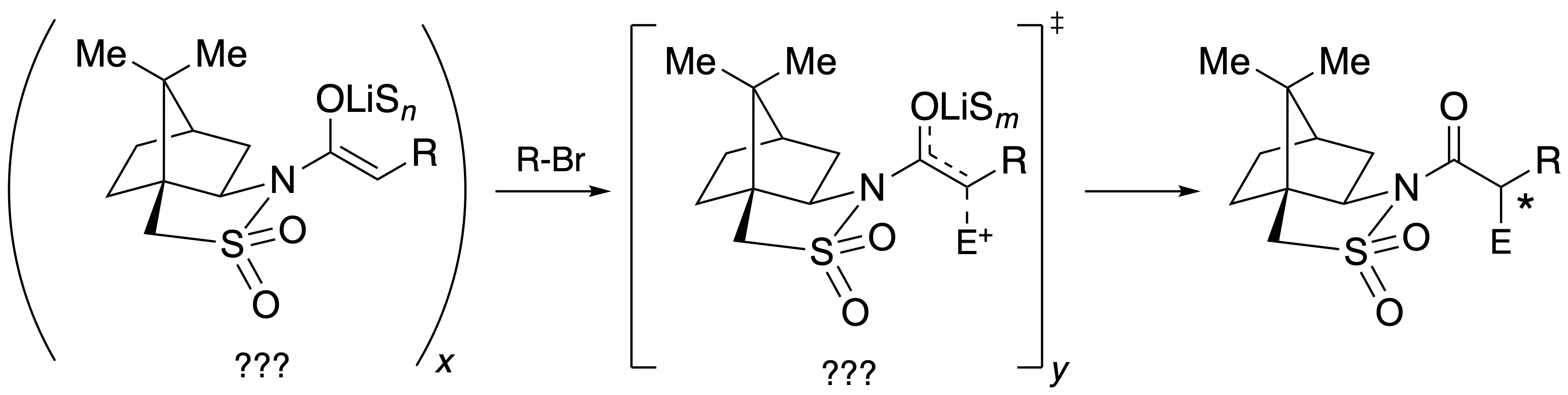
Lithiated Oppolzer Enolates: Solution Structures, Mechanism of Alkylation, and the Origin of Stereoselectivity
Nathan M. Lui, Samantha N. MacMillan & David B. Collum
Accolades
Selected for oral presentation at the Fall 2022 ACS General MeetingAbstract
Camphorsultam-based lithium enolates referred to colloquially as Oppolzer enolates are examined spectroscopically, crystallographically, kinetically, and computationally to ascertain the mechanism of alkylation and the origin of the stereoselectivity. Solvent- and substrate-dependent structures include tetramers for alkylsubstituted enolates in toluene, unsymmetric dimers for aryl-substituted enolates in toluene, substrate-independent symmetric dimers in THF and THF/toluene mixtures, HMPA-bridged trisolvated dimers at low HMPA concentrations, and disolvated monomers for the aryl-substituted enolates at elevated HMPA concentrations. Extensive analyses of the stereochemistry of aggregation are included. Rate studies for reaction with allyl bromide implicate an HMPA-solvated ion pair with a +Li(HMPA)4 counterion. Dependencies on toluene and THF are attributed to exclusively secondary shell (medium) effects. Aided by density functional theory (DFT) computations, a stereochemical model is presented in which neither chelates nor the lithium gegenion serve roles. The stereoselectivity stems from the chirality within the sultam ring and not the camphor skeletal core.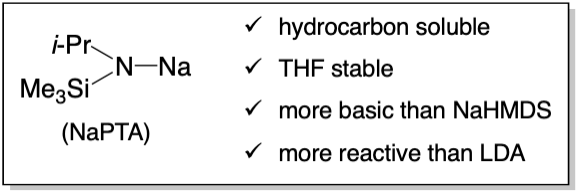
Sodium Isopropyl(trimethylsilyl)amide (NaPTA): A Stable and Highly Soluble Lithium Diisopropylamide Mimic
Yun Ma, Nathan M. Lui, Ivan Keresztes, Ryan A. Woltornist & David B. Collum
Accolades
Featured in the December 2022 installment of “Some Items of Interest to Process R&D Chemists and Engineers” in Organic Process Research & Development.Abstract
The preparation, structure, physical properties, and reactivities of sodium isopropyl(trimethylsilyl)amide (NaPTA) are described. Solubilities at room temperature range from n-heptane (0.55 M), n-hexane (0.60 M), toluene (0.65 M), MTBE (1.7 M), Et3N (3.2 M), and THF (>6.0 M). The half-life to destruction in neat THF is >1 year at 25 °C and 7 days at 70 °C, which compares favorably to 2.5 months and 1.5 days, respectively, for LDA in neat THF. The manuscript focuses on NaPTA/THF solutions. 29Si spectroscopy shows exclusively a mixture of cis and trans stereoisomeric dimers in 0.10–12 M THF in hexane. Density functional theory (DFT) computations suggest a pKb that is intermediate between dimeric sodium diisopropylamide (NaDA) and dimeric sodium hexamethyldisilazide (NaHMDS). Metalations of arenes, epoxides, ketones, hydrazones, alkenes, and alkyl halides show higher reactivities than LDA (kNaPTA/LDA = 1–30). While the rates of arene metalation are high, the lower pKb of NaPTA limits the substrates. Metalation of pseudoephedrate-based carboxamides to form disodiated Myers enolates solves several challenging technical problems.
Spectrochemistry of firefly bioluminescence
Marieh B. Al-Handawi, Srujana Polavaram, Anastasiya Kurlevskaya, Patrick Commins, Stefan Schramm, César Carrasco-López, Nathan M. Lui, Kyril M. Solntsev, Sergey P. Laptenok, Isabelle Navizet & Panče Naumov
Abstract
The chemical reactions underlying the emission of light in fireflies and other bioluminescent beetles are some of the most thoroughly studied processes by scientists worldwide. Despite these remarkable efforts, fierce academic arguments continue around even some of the most fundamental aspects of the reaction mechanism behind the beetle bioluminescence. In an attempt to reach a consensus, we made an exhaustive search of the available literature and compiled the key discoveries on the fluorescence and chemiluminescence spectrochemistry of the emitting molecule, the firefly oxyluciferin, and its chemical analogues reported over the past 50+ years. The factors that affect the light emission, including intermolecular interactions, solvent polarity, and electronic effects, were analyzed in the context of both the reaction mechanism and the different colors of light emitted by different luciferases. The collective data points toward a combined emission of multiple coexistent forms of oxyluciferin as the most probable explanation for the variation in color of the emitted light. We also highlight realistic research directions to eventually address some of the remaining questions related to firefly bioluminescence. It is our hope that this extensive compilation of data and detailed analysis will not only consolidate the existing body of knowledge on this important phenomenon but will also aid in reaching a wider consensus on some of the mechanistic details of firefly bioluminescence.
The elusive relationship between structure
and colour emission in beetle luciferases
César Carrasco-López, Nathan M. Lui, Stefan Schramm & Panče Naumov
Abstract
In beetles, luciferase enzymes catalyse the conversion of chemical energy into light through bioluminescence. The principles of this process have become a fundamental biotechnological tool that revolutionized biological research. Different beetle species can emit different colours of light, despite using the same substrate and highly homologous luciferases. The chemical reasons for these different colours are hotly debated yet remain unresolved. This Review summarizes the structural, biochemical and spectrochemical data on beetle bioluminescence reported over the past three decades. We identify the factors that govern what colour is emitted by wild-type and mutant luciferases. This topic is controversial, but, in general, we note that green emission requires cationic residues in a specific position near the benzothiazole fragment of the emitting molecule, oxyluciferin. The commonly emitted green–yellow light can be readily changed to red by introducing a variety of individual and multiple mutations. However, complete switching of the emitted light from red to green has not been accomplished and the synergistic effects of combined mutations remain unexplored. The minor colour shifts produced by most known mutations could be important in establishing a ‘mutational catalogue’ to fine-tune emission of beetle luciferases, thereby expanding the scope of their applications.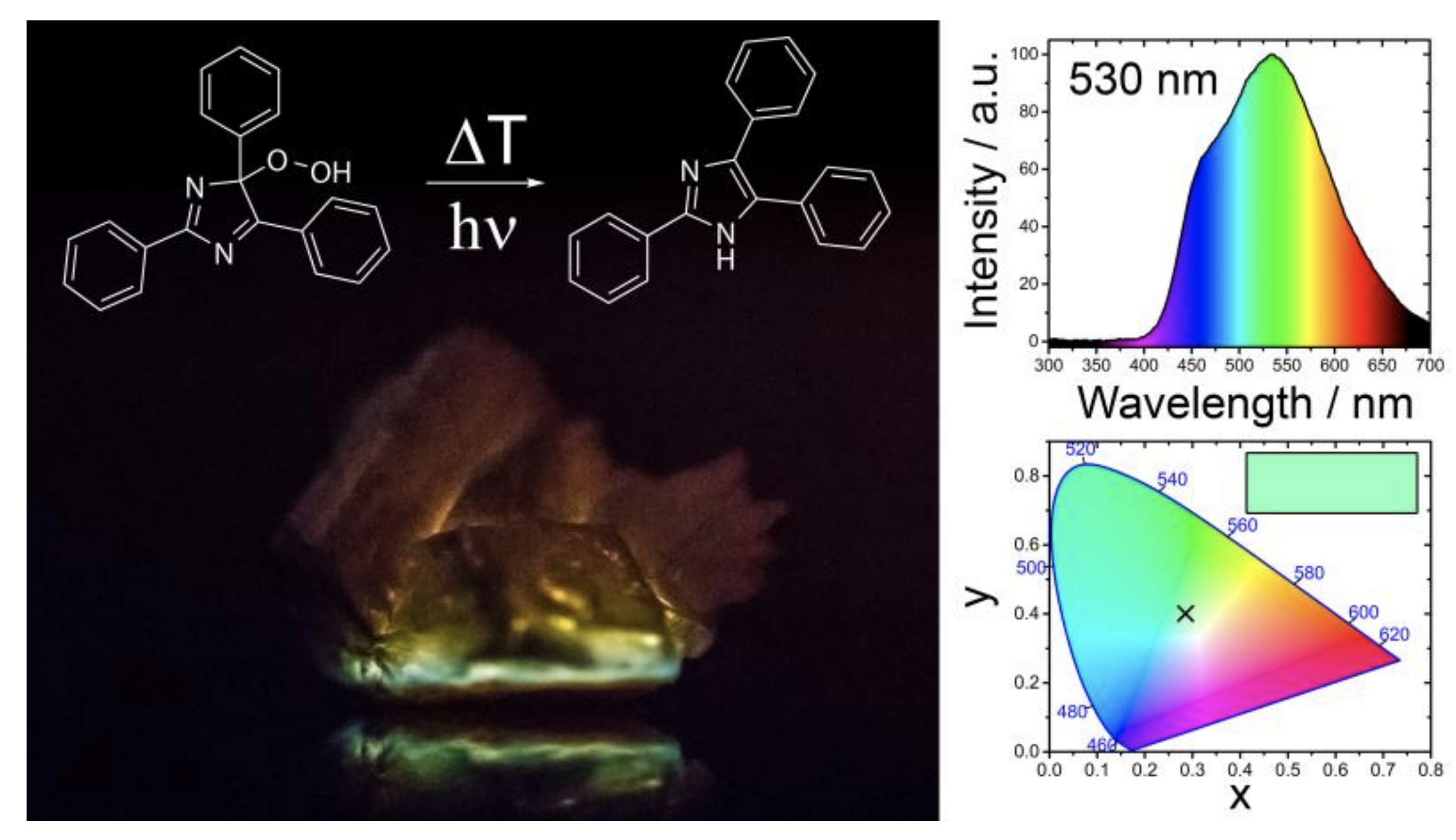
Thermochemiluminescent peroxide crystals
Stefan Schramm, Durga Prasad Karothu, Nathan M. Lui, Patrick Commins, Ejaz Ahmed, Luca Catalano, Liang Li, James Weston, Taro Moriwaki, Kyril M. Solntsev & Panče Naumov
Abstract
Chemiluminescence, a process of transduction of energy stored within chemical bonds of ground-state reactants into light via high-energy excited intermediates, is known in solution, but has remained undetected in macroscopic crystalline solids. By detecting thermally induced chemiluminescence from centimeter-size crystals of an organic peroxide here we demonstrate direct transduction of heat into light by thermochemiluminescence of bulk crystals. Heating of crystals of lophine hydroperoxide to ~115°C results in detectable emission of blue-green light with maximum at 530 nm with low chemiluminescent quantum yield [(2.1±0.1) × 10‒7E mol‒1]. Spectral comparison of the thermochemiluminescence in the solid state and in solution revealed that the solid-state thermochemiluminescence of lophine peroxide is due to emission from deprotonated lophine. With selected 1,2-dioxetane, endoperoxide and aroyl peroxide we also establish that the thermochemiluminescence is common for crystalline peroxides, with the color of the emitted light varying from blue to green to red.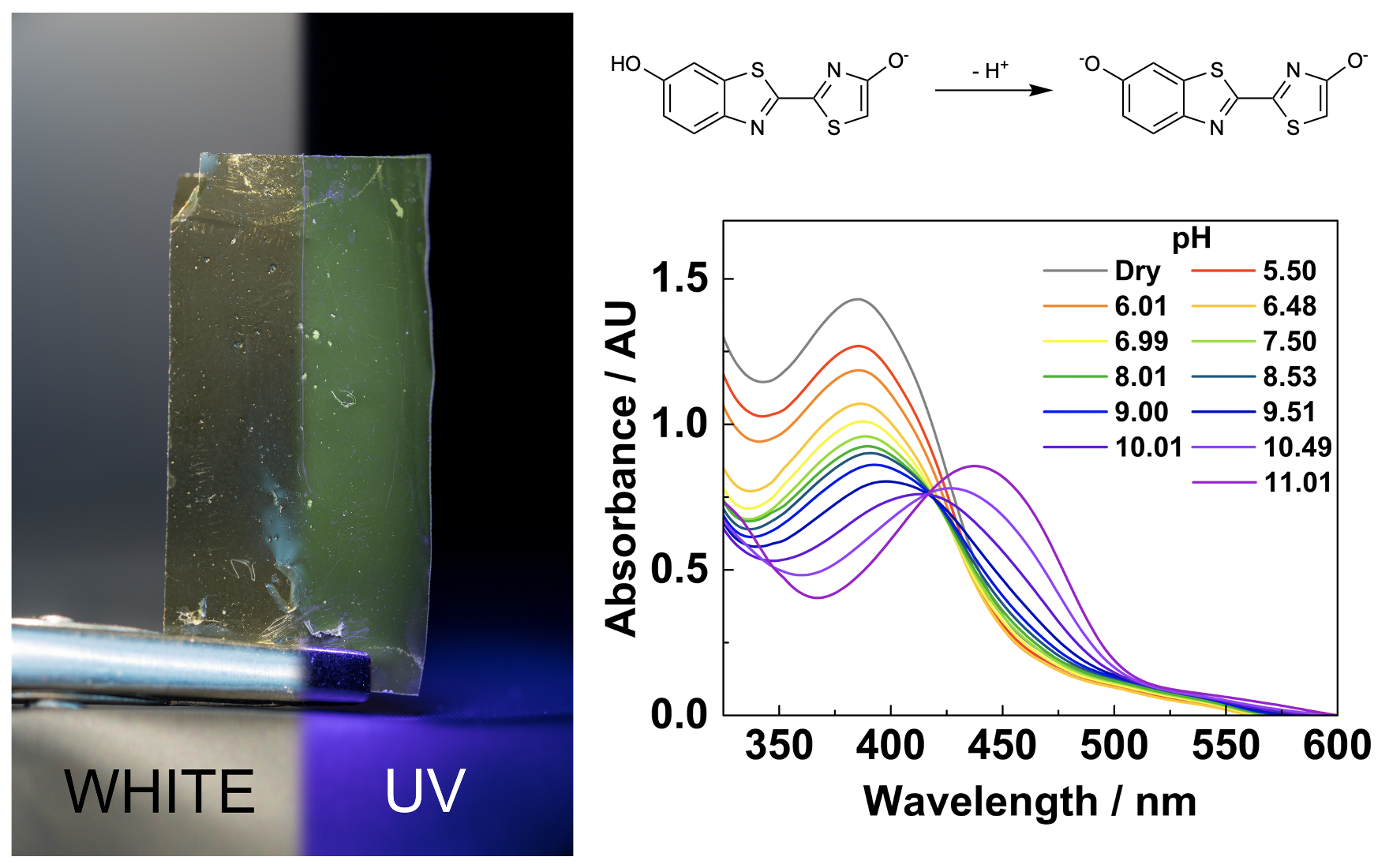
pH-Dependent fluorescence
from firefly oxyluciferin in agarose thin films
Nathan M. Lui, Stefan Schramm & Panče Naumov
Accolades
An abridged version of this paper was selected for an oral presentation at the 5th UAE Undergraduate Research Competition under the title “Humidity responsive luminescent switching in oxyluciferin-agarose thin films as a basis for optical humidity sensors.”Abstract
The yellow-green light emitted by fireflies is one of the most prominent examples of bioluminescence. Firefly oxyluciferin, the emitting molecule, is labile in alkaline solutions, and its structure is strongly affected by solvent polarity and pH. Previous studies have suggested that variations in the active site conditions are likely contributors to the color of bioluminescent emission. Herein, we incorporate firefly oxyluciferin into an agarose matrix to emulate the enzyme active site. Self-supporting, lightweight thin films were fabricated by solution casting and spectroscopically characterized. The previously described acidochromism of oxyluciferin is conserved in the thin films. The bathochromic shift observed in alkaline conditions results from the formation of the oxyluciferin dianion. This study demonstrates an alternative approach to investigating environmental effects on bioluminescent molecules.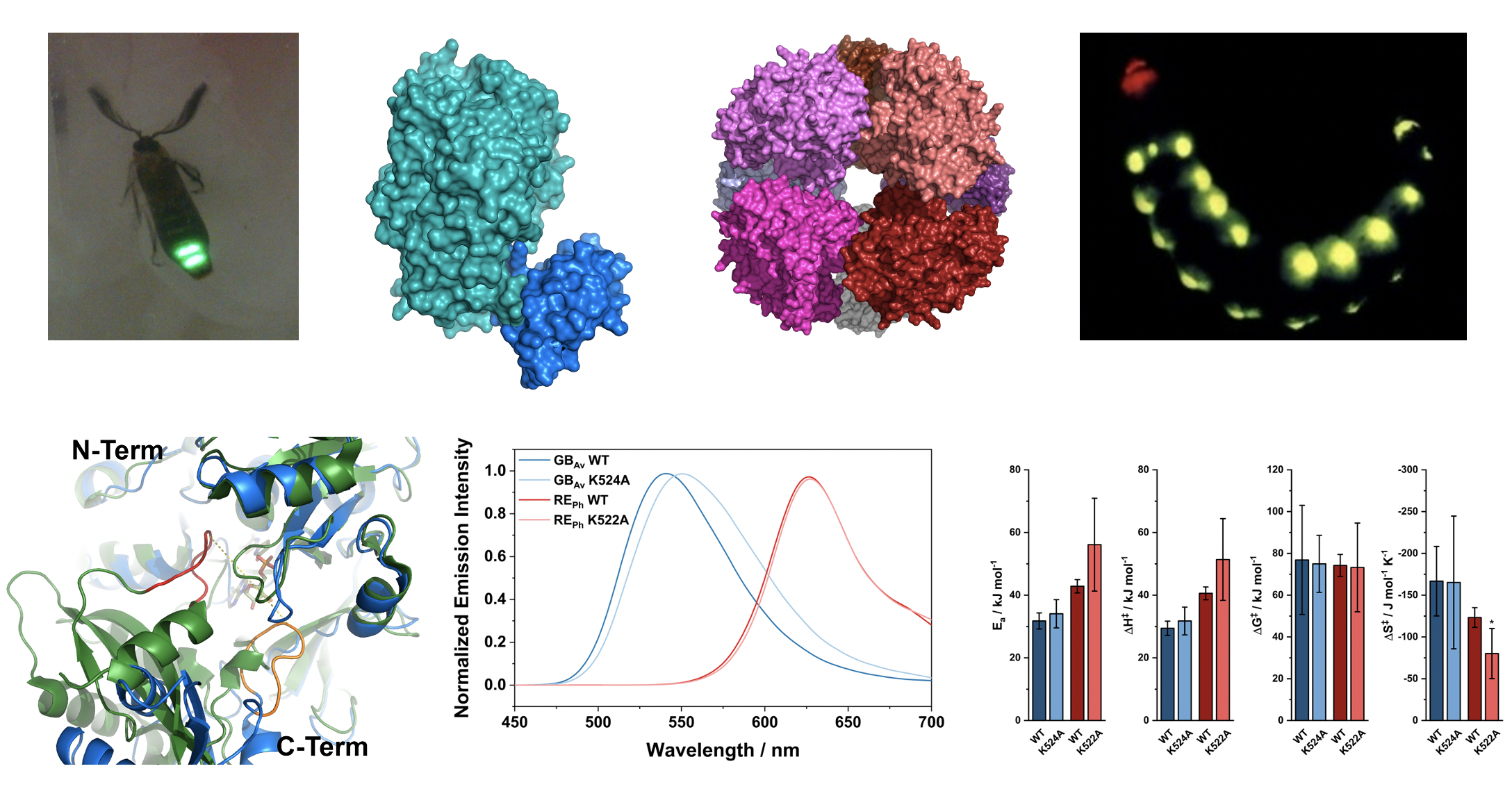
Beetle luciferases with naturally red- and blue-shifted emission
César Carrasco-López, Juliana C. Ferreira, Nathan M. Lui, Stefan Schramm, Romain Berraud-Pache, Isabelle Navizet, Santosh Panjikar, Panče Naumov & Wael M. Rabeh
Accolades
Selected for spotlight talk at 2018 ISBC General Meeting (top abstract in section)Selected for Sci-Mix at the 255th ACS General Meeting (top 20 abstracts in division)
Abstract
The different colors of light emitted by bioluminescent beetles that use an identical substrate and chemiexcitation reaction sequence to generate light remain a challenging and controversial mechanistic conundrum. The crystal structures of two beetle luciferases with red- and blue-shifted light relative to the green yellow light of the common firefly species provide direct insight into the molecular origin of the bioluminescence color. The structure of a blue-shifted green-emitting luciferase from the firefly Amydetes vivianii is monomeric with a structural fold similar to the previously reported firefly luciferases. The only known naturally red-emitting luciferase from the glow-worm Phrixothrix hirtus exists as tetramers and octamers. Structural and computational analyses reveal varying aperture between the two domains enclosing the active site. Mutagenesis analysis identified two conserved loops that contribute to the color of the emitted light. These results are expected to advance comparative computational studies into the conformational landscape of the luciferase reaction sequence.Presentations
Upcomming presentations
- American Chemical Society National Meeting | San Diego, SD, USA
“Transfer learning approaches for reaction product prediction in the low-data regime." Oral presentation, Mar 2025. - Computer Aided Drug Discovery Gordon Reserach Conference | Portland, ME, USA
Title TBD, Poster, July 2025.
Conference presentations and academic seminars
- Lilly Postdoc Summit | Indianapolis, IN, USA
“A deep learning approach to mechanism-aware reaction product prediction." Spotlight talk and poster, 10-11 Oct 2024. - Lilly Global Computational Chemistry Summit | San Diego, CA, USA
“A deep learning approach to mechanism-aware reaction product prediction." Research highlight and selected poster, 23 Sep 2024. - Cornell University | Ithaca, NY, USA
“Structure-selectivity principles underlying the alkylation of Oppolzer's camphorsultam enolates." Doctoral dissertation defense, 7 July 2024. - American Chemical Society National Meeting | Chicago, IL, USA
“Structure and mechanism of the alkylation of Oppolzer’s camphorsultam-derived enolates." Selected talk, 21 Aug 2022. - Graduate and Postdoc Spring Seminar Series | Ithaca, NY, USA
“Structure and Mechanism of Lithium Enolates of the Oppolzer Sultam.” Seminar, 15 Apr 2022. - 20th International Symposium on Bioluminescence and Chemiluminescence | Nantes, France
“The active site microenvironment determines the color of emission in beetle luciferases.” Research spotlight talk and selected poster, 29 May 2018. - American Chemical Society National Meeting | New Orleans, LA, USA
“Approaching the color problem of bioluminescence: Contributions of the active site microenvironment to the emission of red and green luciferases.” Sci-Mix poster presentation, 19 Mar 2018. - Environmental & Materials Science Symposium | Abu Dhabi, UAE
“Bioluminescence in Nanotechnology: Characterization of two novel luciferases for applications in emerging nanobiotechnologies.” Poster presentation, 6 Dec 2017. - American Chemical Society Asia-Pacific International Chapters Conference | Jeju Island, South Korea
“CellPlus: Paving the way for artificial organelles by the enzyme-instructed self-assembly of guanosine derivatives.” Poster presentation, 6 Nov 2017. - Middle East Molecular Biology Sources (MEMBS) Annual Congress | Abu Dhabi, UAE
“Structural insight into the mechanism of a blue-shifted green-emitting luciferase.” Selected oral presentation, 3 Nov 2017. - NSF-REU: PR-CLIMB symposium | San Juan, Puerto Rico, USA
“CellPlus: Paving the way for artificial organelles by the enzyme-instructed self-assembly of guanosine derivatives.” Poster presentation, 27 July 2017. - 5th Annual UAE Undergraduate Research Competition | Abu Dhabi, UAE
“Humidity responsive luminescent switching in oxyluciferin-agarose thin films as a basis for optical humidity sensors.” Selected oral presentation, 8 May 2017. Abu Dhabi, UAE. - 9th Annual International Workshop on Advanced Materials | Ras Al Khamiah, UAE
“Humidity responsive luminescent switching in oxyluciferin-agarose thin films as a basis for optical humidity sensors.” Poster presentation, 20 Feb 2017. - 2nd Annual Crystal Growth Symposium | Shanghai, China
“Crystallization of NiSO4 polymorphs: The importance of temperature, saturation, and solvent polarity in crystallization” Oral presentation, 4 May 2016.
